Diffuser Selection
Initial task
As mentioned last week I’m currently being asked to look at two projects, a Biopharm for GlaxoSmith Kline (GSK) and the re-fit of some steam plant at the London School of Hygiene and Tropical Medicine. I get the impression that Bryden Wood were hoping to initially fill my time with the steam plant project, however, I won’t be able to get involved with this until Wed at the earliest. Therefore I’m having a bit of a slow start, which is no bad thing after the last few months on site. My task to date has been to look at possible diffuser solutions (air supply) in a number of rooms in the Biopharm facility, which will ultimately feed into the specification for the main contractor. I’ll start with a few paragraphs stolen from the GSK website:
Firstly what are Biopharmaceuticals?
Biopharmaceutical products are medicines engineered by scientists and manufactured by living organisms to treat specific ailments. Biopharmaceuticals often target specific cellular functions and tend to be more potent than chemically synthesised pharmaceuticals. Today, 16% of all pharmaceuticals are biologic in nature – treating a wide range of disease areas.
What does Biopharmaceutical Manufacturing involve?
All biopharmaceuticals are manufactured in the same way. Closed, controlled reactors cultivate cells or organisms to produce large quantities of the medicinal product. During cultivation, the reactors are fed with nutrients and its temperature, pH, and aeration are controlled.
Once enough of the product has been produced, the reactor contents are harvested.
Further processing isolates, purifies, and stabilizes the bulk product. It is frozen and stored until formulation and dispensing into its final dosage form used by our patients.
To keep production free from unintended contamination by naturally occurring microbes, manufacture is mostly executed in closed, clean, and sterile equipment. Cleanroom facilities and personnel gowning (similar to hospital operating theatres) provide additional assurance of product quality.

GMP classifications in terms of allowable particle numbers and size per metre cubed. See paragraph below.
What impact does this have on MEP?
It is this final paragraph leads to a requirement for varying levels of cleanliness in rooms depending on their use. The specification for cleanliness is via a standard called Good Manufacturing Practice (GMP).
The rooms I am looking at require a GMP classification of either C or D. This is achieved by increasing the number of air changes per hour (ACH) to a minimum of 10 for class D rooms and 20 for class C rooms. Additionally specific bag filters are use in the Air Handling Units (AHUs) and HEPA filters inserted into the system (see Fran’s blog on ductwork testing).
What’s the problem / why have I got involved?
The strategy for meeting the ventilation requirements in the class D rooms is for high level supply and extract. Unfortunately there doesn’t appear to be enough ceiling space to achieve this; the reactors that cultivate cells are fed by pipework from above, in addition the ceiling is up to 5m high in places and the lighting is being supplied with LEDs, which leads to large emitter arrays being required. The knock on of this is that there is little space for both supply diffusers and extract grilles in the ceiling. We are therefore looking to provide high level supply and low level extract.
My task has been firstly to look at what diffuser options meet our requirement. I approached this in the following manner:
- Identify what the air velocity requirements are for the clean rooms – .15 m/s to .45 m/s for class D rooms. (CIBSE guide B)
- Use the ACH values and room volumes to work out what the required room air flow rates were. The assumption was made that the GMP requirement would be in excess of the heating / cooling requirement.
- Use manufacturer’s data sheets to narrow down the number of products that meet our requirement (figure 1 and 2). The main driver for this was the “throw”. Where throw is the distance from a diffuser to where the velocity of the air jet drops to 0.5 m/s. The throw that is achievable for a given diffuser will predominantly depend on its design, its use (heating, cooling or ventilation) and the flow rate of air through it. Most manufacturer’s don’t give a distance for “throw” in their data sheets, but do give a distance to the 0.2 m/s isovel (line of constant velocity), which is actually more use in my circumstance.
- A secondary consideration was ensuring that the noise rating of the diffuser was acceptable along with the pressure drop across the diffuser.
This has allowed me to scope a couple of potential solutions. I am now in the process of approaching suppliers to confirm the type of diffuser and suggested layouts. We’ll then be able to put this back into our model to see how it impacts other services and eventually write it into the specification.
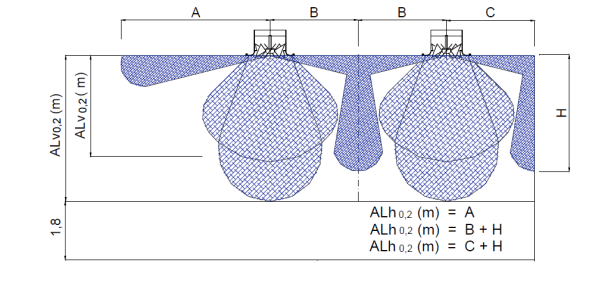
Figure 1. This figure is taken from a manufacturer’s guide and shows both the vertical and horizontal at a distance where the jet velocity has dropped to .2 m/s. The darker blue cloud indicates how the air jet would move when the diffuser is set up for isothermal or cooling conditions and the coanda effect is being utilised. The lighter colour bubbles indicate the blades in the diffuser being at a steeper angle to force warm air down into the occupied space. My room is 5m high and has 3m between diffusers, therefore ALv is 5m and ALh is 6.5m (the occupied zone is to the floor as the requirement is for cleanliness as opposed to the 1.8m indicated above for someone standing).
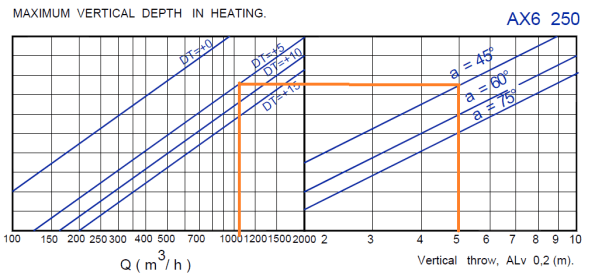
Figure 2. From figure 1 it is possible to calculate ALv and ALh. In my case ALv is 5m. I’ve used this with a blade angle of 45 degrees and a heating delta t of 5 degrees to calculate that one AX6 250 diffuser would need to be pushing through just over 1000 m cubed per hour to meet these conditions. This is then checked against the capability of the diffuser to see if it works, if it doesn’t either the blade angle can be changed or another diffuser has to be selected.
The next stage is to try and work out the low level return will work; part of the design brief is that GSK want the facility to conjure images of “ the Google of Pharmaceuticals” and for the process to be transparent. This is resulting in most of the internal walls being glass – not entirely sure how we’re going to turn glass walls into return plenum’s.
Rich,
Sounds interesting…and thanks for the reference to my blog. Have fun designing that glass plenum. Will be interesting to see how it turns out.
Fran,
I think (and also hope) the glass plenum is going to be VE’d. I’ve already asked the question as to how are we going to clean them – won’t look so Google when there’s dirt streaks running up the inside pane next to the ventilation extract. Other problems include how to mount the frames of the extract grilles and ensuring the joints between the glass panes don’t leak. What started out in the clients mind as a sleek wall becomes a bit of an abortion.